
Sources of Nitrogen Oxides

22.16 Emissions of sulfur dioxide and nitrogen oxides react with the

Human Sources of Nitrogen Oxides: At normal temperatures the oxygen and

Main national sources of oxides of nitrogen, 2003-04
Oxides of nitrogen have varied chemical behaviour because of their various

such as oxides of nitrogen, carbon monoxide, and fine particle matter.

oxides of nitrogen (NOx)
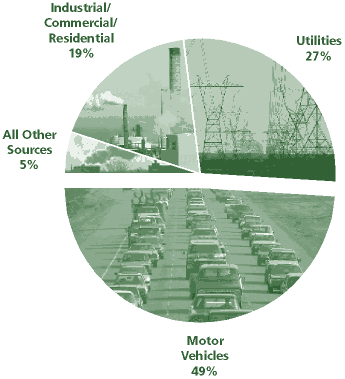
Oxides are motor vehicles, electric utilities, and other industrial,

oxides of nitrogen

Oxides of Nitrogen + Volatile Organic Compounds + Heat & Sunlight

both nitrogen oxides

Normal combustion emits nitrogen oxides, because nitrogen reacts with oxygen

Annual emissions, Sydney Region: Oxides of nitrogen (NOx) -

Chart of Nitrogen Oxides

"Nitrous oxide" can be properly used to refer to any oxides of nitrogen,

is created by a chemical reaction between sunlight, oxides of nitrogen,

gases' such as methane and oxides of nitrogen in the atmosphere.

89.112 Oxides of nitrogen, carbon monoxide, hydrocarbon, and particulate

The advantages of using DME are ultralow emissions of nitrogen oxides (NOx),

(3) Great quantities of sulfur dioxide and nitrogen oxides mix with the